Sodium Thiosulphate and Hydrochloric Acid
Sodium thiosulphate 10. Add 10 ml of hydrochloric acid and 2 g of potassium iodide stopper shake and keep in dark for 15 min.
Rate Of Reaction Of Sodium Thiosulfate And Hydrochloric Acid Flinn Scientific
Hydrofluoric acid 40.

. Since 2011 serving entrepreneurs small and medium-sized businesses large corporations Governments and procurement specialists from the private and public sectors. When it is almost all gone you add some starch solution. Sodium chloride aqueous solution 10.
Uses for Hydrochloric Acid A highly corrosive mineral acid hydrochloric acid has many industry uses making it known as a workhorse chemical. Buy Sodium Hypochlorite from Trusted UK Chemical Suppliers. Sodium bisulphite aqueous solution 10 Sodium carbonate aqueous solution 10.
Add 100 ml of water to the above mixture and titrate with sodium thiosulphate using. Add 2 mL of 6 NsHCl. Sodium hypochlorite solution is produced on an enormous scale throughout the world and is a by-product of chlorine manufacture.
Isopropyl Alcohol IPA Isophthalic Acid. Pipette 200 mL sample into the flask discharging the sample under the surface of solution. Sulphuric acid concentrated 98.
Add distilled water and bring the volume to 20 mL. Sorbitol ˈ s ɔː r b ɪ t ɒ l less commonly known as glucitol ˈ ɡ l uː s ɪ t ɒ l is a sugar alcohol with a sweet taste which the human body metabolizes slowly. In the production of different types of chlorides and metals.
Standard iodine solution 0025N Standard sodium thiosulphate solution 0025N Starch solution. Polyethylene Terephthalate PET--Chemical Compatibility G PET exhibits good resistance to attack F PET exhibits fair resistance to attack X PET. Dissolve 0125 g of accurately weighed potassium dichromate in 25 ml of water present in a 250 ml erlenmeyer flask.
It is a by-product of chlorine manufacture along with sodium hypochlorite and sodium hydroxide. The reaction is marked to an end by the formation of this product. You add the last few drops of the sodium thiosulphate solution slowly until the blue.
As the sodium thiosulphate solution is run in from a burette the colour of the iodine fades. You can buy sodium hypochlorite online at ReAgent a leading chemical manufacturer with almost 50 years of renowned industry experience. Hydrochloric acid is colorless sodium thiosulphate is a white crystal The two will react to form sodium chloride salt sulfur water and sulfur dioxide gas sulphur is solid with intense yellow color.
Most sorbitol is made from potato starch but it is also found in nature. Sulphuric acid aqueous solution 2. Hydrochloric acid aqueous solution 36.
Sodium nitrate aqueous solution 10. Hydrochloric Acid 10 G concentrated X Hydrofluoric Acid 5 G 50 X Hydrogen Peroxide 3 G 30 G Hydroquinone pure solid G IronIII Nitrate pure solid G Isooctane pure liquid G Isopropyl Alcohol pure liquid G. Replenishes depleted thiosulphate stores by acting as sulfur donor necessary for the conversion of CN-O to thiocyanate through the action of sulfur transferase enzyme rhodanese.
Ingredient Depot delivers chemicals ingredients and raw materials in North America wide. Determine the enthalpy change during the interaction hydrogen bond formation between acetone and chloroform. To set up simple Daniell cell and determine its.
Hydrochloric acid 30 S S Methylsulfuric acid S S Hydrochloric acid 35 S S Milk S S Hydrocyanic acid S S Mineral oils S U Hydrocyanic acid saturated S S Molasses S S Hydrofluoric acid 40 S S Mustard prepared S S Hydrofluoric acid 60 S S Hydrofluoric acid 75 S S N Hydrogen 100 S S Naphtha O U Hydrogen bromide 10 S S Naphthalene S U Hydrogen. In the refinement of ore. Linear Alkyl Benzene LAB Linear Alkylbenzene Sulfonic Acid.
This can be observed by the change in color and the disappearance of the red mark as the products are. Measure from a burette 10mL of iodine into a 500 mL flask. It can be obtained by reduction of glucose which changes the converted aldehyde group CHO to a primary alcohol group CH 2 OH.
Standardization of sodium thiosulphate. Thermochemistry Viva Questions with Answers. Determine the enthalpy of neutralisation of hydrochloric acid with sodium hydroxide solution.
We have a full range. This reacts reversibly with iodine to give a deep blue starch-iodine complex which is much easier to see. Neutralization of hydrochloric acid formed when chlorine gas reacts with water in the airways.

Sodium Thiosulfate Hydrochloric Acid Experiment Video Lesson Transcript Study Com
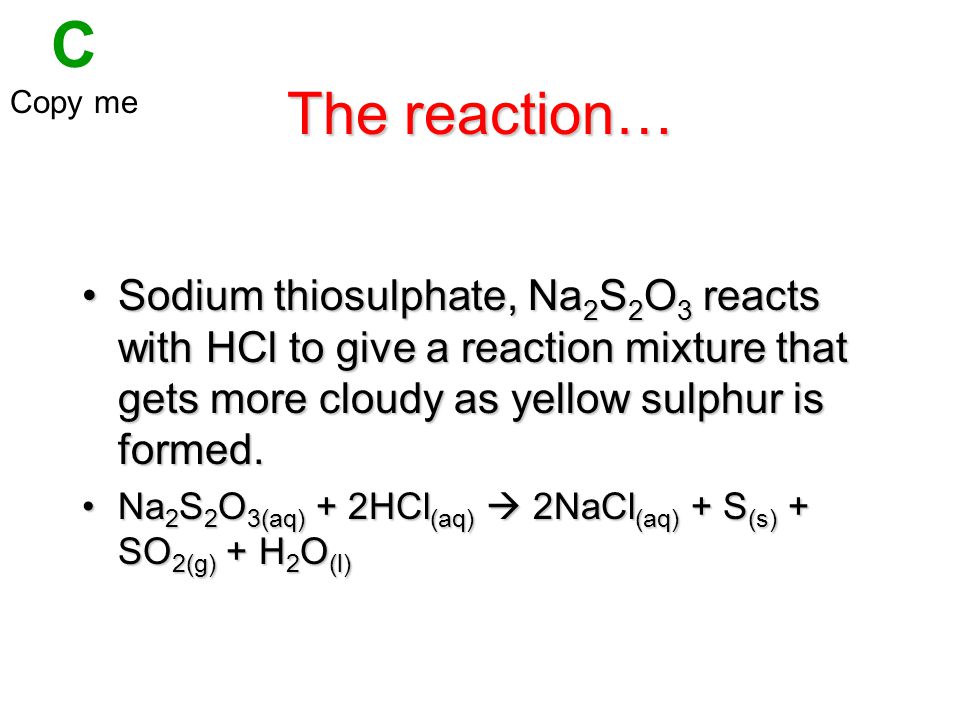
Reaction Of Sodium Thiosulphate And Hydrochloric Acid Ppt Video Online Download

How To Balance Na2s2o3 Hcl Nacl H2o S So2 Youtube

Rate Of Reaction Of Sodium Thiosulfate And Hydrochloric Acid Youtube
0 Response to "Sodium Thiosulphate and Hydrochloric Acid"
Post a Comment